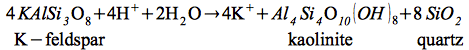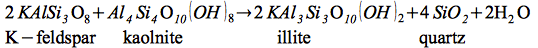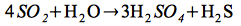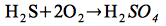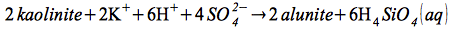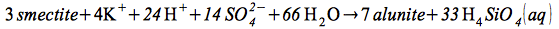GeoPeP 2011/2012
Disscusion
The presented results provide implications on different types of alteration of local volcanic rocks which turned out to be individually specific for the four studied locations.
Assuming that Al and Ti are relatively immobile compared to Na and K, this ratio combined with the SiO2 content in wt%, allows for four main types of alteration to be distinguished, as seen in figure 20. On the one hand, a) a low-grade alteration characterized by a successive depletion of Na and K relative to Al and Ti is observed, transitioning towards a so called b) mild to advanced argillic alteration which is even more depleted in Na and K. Both types exhibit a comparatively constant SiO2 content. With a further depletion in Na and K, two trends of c) silicification and d) Fe-hydroxide enrichment can be distinguished.
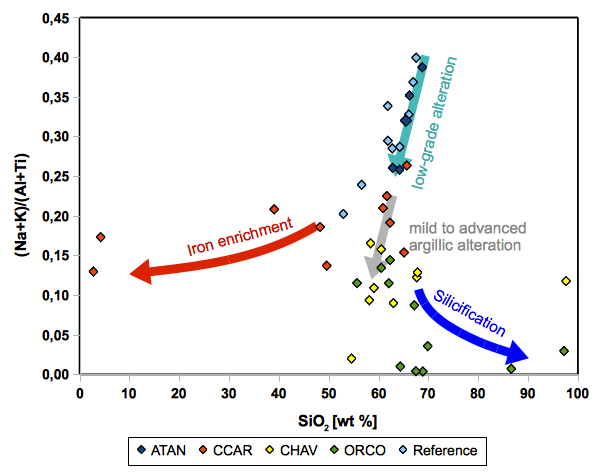
Fig.20: Different alteration Trends
a) low-grade alteration associated with zeolithisized ignimbrites
Lomada Atansa and some Carhuarazo samples fall onto the trend of magmatic differentiation represented by the fresh reference values. However, for differentiation trends, REE and HFSE should increase with increasing differentiation and SiO2. Anyhow, in these samples, REE and HFS elements remain quite constant (see fig. 3), indicating that the successive depletion in Na and K results from an alteration process rather than a differentiation process. In addition, all of the ATAN and CCAR samples have a chemical index of alteration above 0,6 and thus most are distinctly higher than the reference samples (Brandmeier et al., 2012). Therefore, we consider that these samples are also altered and show a low-grade alteration as a kind of pre-stage to the argillic alteration described in Hennig et al. (2008).
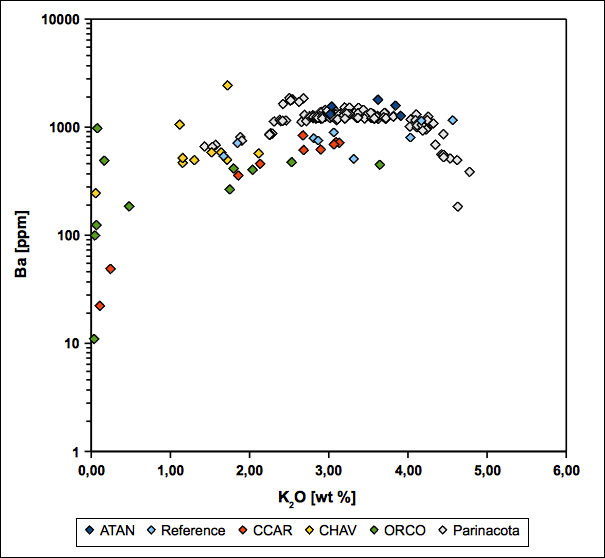
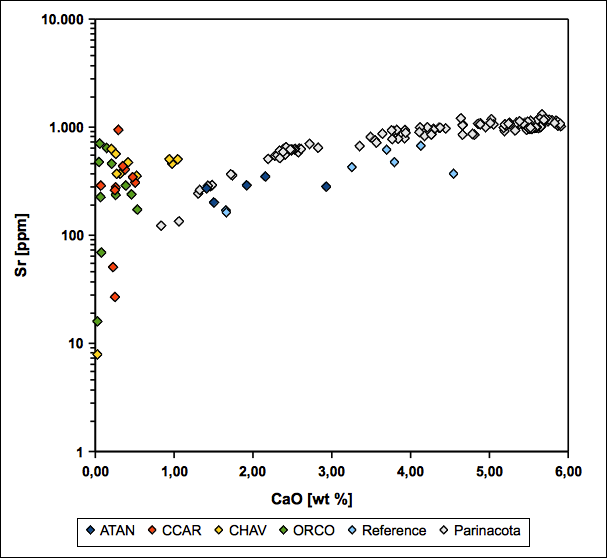
Regarding Ba-K2O and Sr-CaO variation diagrams, samples show similarities to a fresh Parinacota volcano reference (see fig. 21 and 22). This coincides with the possible occurrence of relictic feldspar and K-bearing illite as an alteration product of K-feldspar as indicated by EMP and XRD. Whereas the feldspar relicts remain relatively rich in alkali elements, the surrounding matrix tends to be depleted in this regard. This implies a fixation of alkali elements in the feldspar as long as it is thermodynamically stable.
For some Lomada Atansa samples a high-grade hydrothermal alteration can be inferred from the occurrence of pore-filling zeolithe (see figure 23), likely grown due to the presence of circulating fluids in the former pore space.
|
|
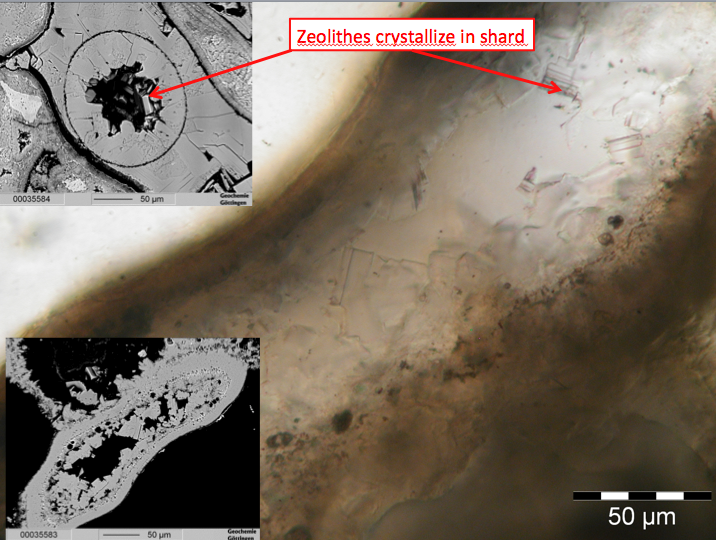
Fig.23: Porefilling zeolithes in ATAN-samples (from Brandmeier et al., 2012)
b) mild to advanced argillic alteration
With successive depletion in Na and K, a mild argillic alteration causes the formation of several clay minerals. Signs of this alteration type are observed in samples from Cerro Chavina, Cerro Orconcchocha and Carhuarazo.
Mineral assemblages including kaolinite, illite, and quartz can be attributed to the breakdown of feldspar due to the infiltration and circulation of pore fluids under relatively low acidic conditions.
Kaolinite formation:
| |
(1) |
Illite formation:
| |
(2) |
This involves the leaching of alkali elements and the relative enrichment of SiO2 and Al2O3.
Argillic alteration according to Hennig et al. (2008) also includes smectite in the characteristic assemblage, which might also occur in the locations presented in this study, but has yet to be identified.
The occurrence of jarosite and alunite as members of the alunite group minerals and barite, coinciding with high sulphate contents, indicates the presence of high-sulfidation fluids. A resulting insolubility of barite also provides a reasonable explanation for the relative immobility of barium derived from the breakdown of K-feldspar and biotite (see fig. 21) (Hennig et al. 2008). In addition, where CaO becomes depleted during dissolution of plagioclase, Sr remains constant due to fixation within kaolinite and alunite group minerals (see fig. 22). Thus, several samples can be assigned to the advanced argillic alteration, based on fig. 10 in Hennig et al. (2008).
According to Hennig et al. (2008), we suggest a magma chamber releasing volatile components like CO2 and SO2.2. In the presence of water, the latter one partly disproportionates to sulfuric acid and H2S gas (Dill, 2000; Henley and Ellis, 1983).
Disproportionation reaction:
| |
(3) |
A boiling fluid composed of H2S, CO2 and SO2 ascends until H2S gets oxidized in contact with the atmosphere, forming sulfuric acid, according to Dill (2000):
Oxidation of H2S:
| |
(4) |
The sulfuric acid descends and acidifies the oxidizing steam-heated groundwater, producing acidic fluids of pH > 2 and temperatures around 100°C, causing an advanced argillic alteration and the formation of alunite group minerals (Dill, 2000; Hennig et al., 2008).
Alunite formation from kaolinite:
| |
(5) |
Alunite formation from smectite:
| |
(6) |
Iron supply for jarosite formation can be provided by the breakdown of Fe-bearing minerals such as hematite or biotite.
c) silicification
Different degrees of silicification are a common observation in several samples, especially at Cerro Chavina and Cerro Orconcchocha. Initial signs for this process in context with feldspar breakdown and related microcrystalline quartz formation, can be found in samples which are thought to have undergone mild to advanced argillic alteration.
A massive pervasive silicification is represented in samples CHAV-11-17 and ORCO-11-01-1 which comes along with a widespread textural destruction and the replacement of phenocrysts and groundmass by a fine-grained mixture of quartz that is again affected by a coarsening recrystallization resulting in the formation of euhedral quartz crystals (see fig. fig. 7). This process can be retraced in samples ORCO-11-01-1 to ORCO-11-01-3 as indicated by ion microprobe results (see fig. 6 and fig. 5), whereas ORCO-11-01-1 shows the highest degree of silicification.
In addition, silicification is supported by the dominance of quartz and amorphous silica in several XRD spectra (e. g. see fig. 19).
With respect to XRF results, a leaching of mobile components such as Na2O and K2O, plus relatively immobile Al2O2, in the later stages is observed in context with the SiO2 enrichment.
Regarding samples ORCO-11-01-1 to ORCO-11-01-3, a primary step of mild to advanced argillic alteration (see above) is followed by a second stage of high-grade hydrothermal alteration. The successive loss of Al2O3 can be explained by the infiltration and circulation of a fluid characterized by even higher temperatures (200 to 300°C; Stoffregen, 1987) and pH values below 2, resulting in an increasing solubility of Al (Henley and Ellis, 1983). These extraordinary low pH values can be inferred from an extensive production of sulphuric acid after equation (3). The decrease of Sr and Ba suggest that these aggressive conditions finally lead to the instability of phases like kaolinite, alunite and barite, which formerly functioned as a carrier for Sr and Ba (see fig. 21 and fig. 22).
In this context an assumption can be made concerning the trace elemental pattern of CHAV-11-17 and ORCO-11-01-1, which exhibit exceptional low REE enrichments and in particular low LREE. Regarding monazite as a known carrier of LREE, an analogue breakdown would result in the existing pattern.
d) Fe-hydroxide enrichment
While several samples are affected by SiO2 enrichment (silicification), Carhuarazo samples show a tendency towards SiO2 depletion that is accompanied by a strong enrichment in Fe2O3 as indicated by XRF data (see fig. fig. 2).
We assume an extensive leaching of the andesitic to rhyodacitic host rock by ascending steam-heated acidic (pH < 3) fluids released from an underlying magma chamber. When the uprising Fe-enriched fluids get in contact with the atmosphere and meteoric waters, this results in the precipitation of Fe-hydroxides and –oxides such as hematite (Fe2O3), goethite and limonite (FeO(OH)), forming massive crusts at the surface. Otherwise, with respect to iron coatings of silicate phases (see fig. 12), Fe-hydroxides and –oxides might precipitate within porous fine-grained granular scree sediments. Taking in account that Carhuarazo was a target of mining for silver and gold by the Spanish conquerors, whereas these elements are enriched by hydrothermal fluids, a hydrothermal leaching as a source for the iron seems to be likely, as well.
© Christian Hansen, Andres Höweling, Kai Nitzsche, Tammo Ohlendorf 2012